Antibiotics work in many ways. One of the most common ways they kill bacteria is by binding with a bacterial enzyme - a type of protein that carries out reactions in bacteria. This stops the bacteria from growing and causing infections in humans.
However, bacteria have evolved to resist the action of these antibiotics in a number of ways. This could be by damaging the antibiotics so that they are unable to bind with their target enzyme, or by changing the structure of the target enzyme itself to protect it from the antibiotic.
IOI chemists use a technique called crystallography to look at the three-dimensional structure of bacterial enzymes to understand these interactions, and use this information to develop new antibiotics.
We caught up with Dr Helen Smith, a Postdoctoral Research Associate at the IOI, who uses crystallography to develop new antibiotics.
“The first step in crystallography is producing crystals.” says Helen. So, what are crystals?
Crystals are tiny three-dimensional solids of bacterial enzymes which capture them in one conformation, which means one specific shape. Researchers can then get a clear view of the enzyme from lots of angles, which helps them find out its structure and understand how an antibiotic molecule binds to it.

Bacterial enzymes are made and purified in the lab, mixed with different compounds, including different antibiotics, in tiny droplets. Over time the enzymes form solids that separate out of these droplets as crystals, which then need to be collected.
“Fishing out the crystals is quite tricky, we use tools to collect them using a microscope as guidance, almost like doctors would during surgery. It can get quite competitive in the lab – every year we crown a fishing champion!” says Helen.
Crystals are sent to Diamond Light Source, where they are placed inside an X-Ray beam which is scattered by the molecules within the crystal, producing a diffraction pattern which is recorded by a computer. The diffraction pattern is specific to each crystal as it depends on the three-dimensional conformation of the protein molecules within it and how they are arranged with respect to each other.
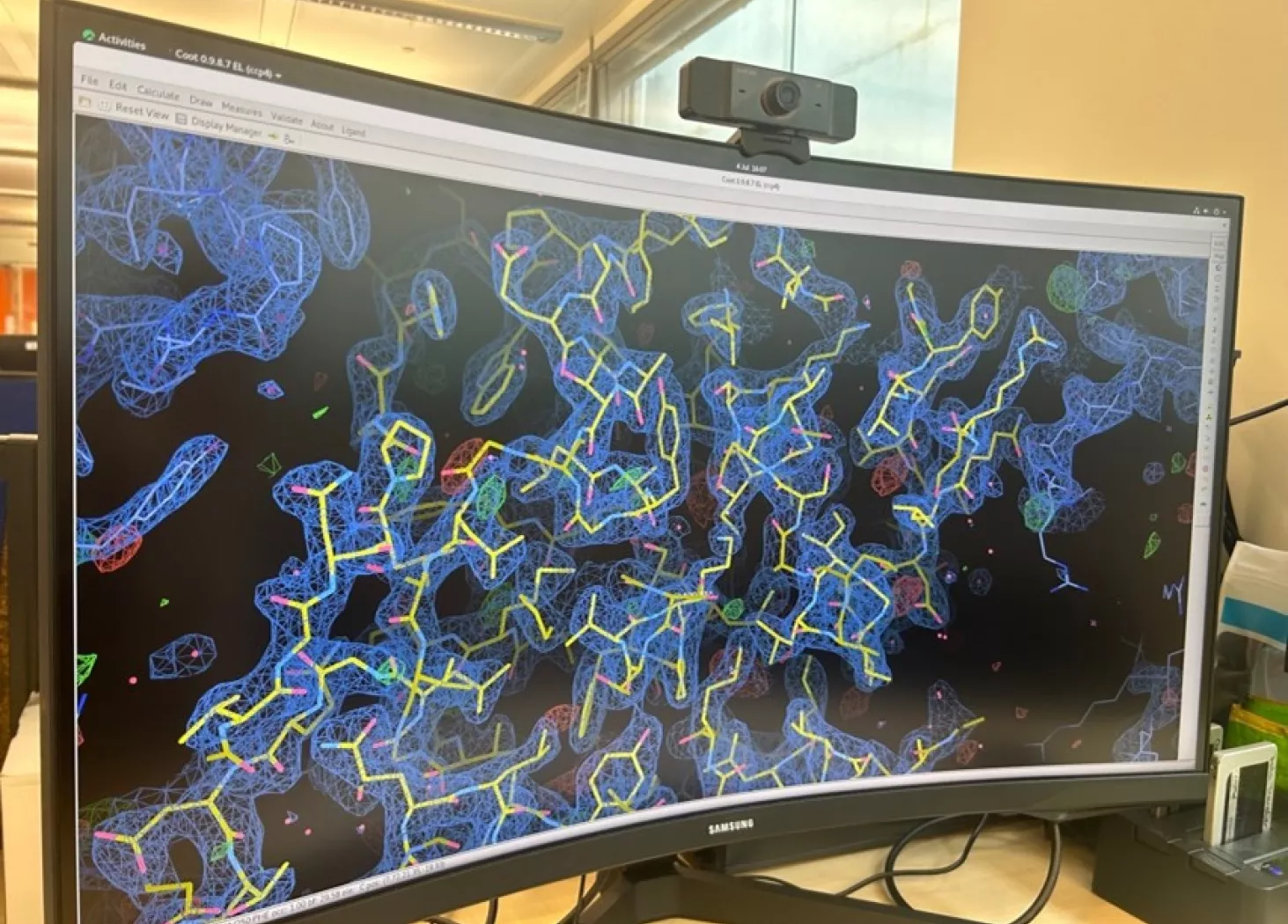
The diffraction pattern produced from each crystal is analyzed and transformed by supercomputers so researchers can see the three-dimensional structure of the bacterial enzyme, and any compound bound to it.
This data provides extra insight for researchers, beyond what they can infer from carrying out experiments in the lab.
Seeing the interaction between a bacterial enzyme and molecule provides valuable insights about how we can refine the compound structure to improve the binding and maximize its effectiveness as an antibiotic.”

Using her training in synthetic chemistry and biochemistry, Helen has enjoyed expanding her capabilities in crystallography supported by key members of the Schofield lab, including Dr Mark Allen and Dr Stephen Marshall.
Our work adds to the wider knowledge of how bacterial enzymes work, and the more information that the wider scientific community has, the closer we get to finding new and improved antibiotic treatments.

