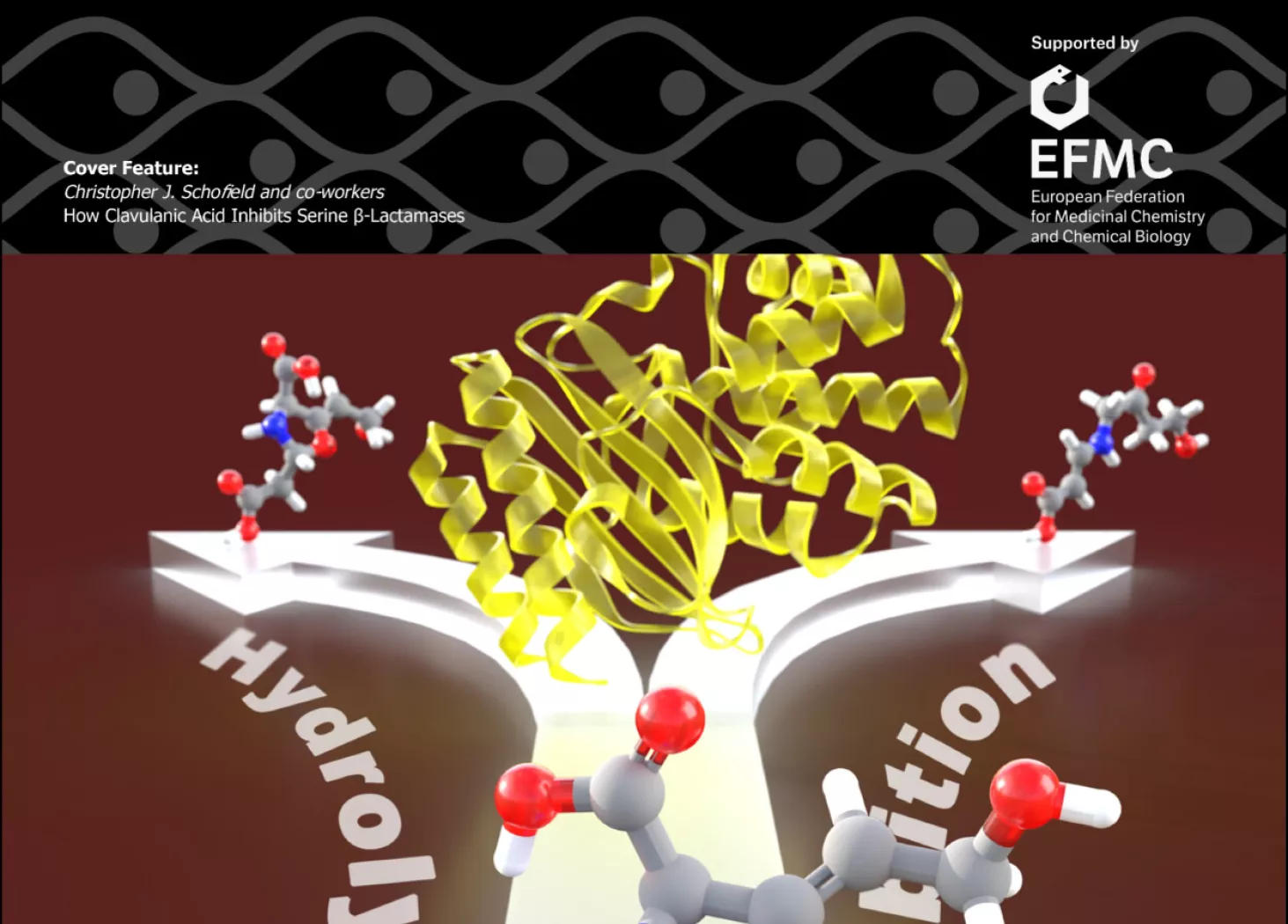
Clavulanic acid is a densely functionalized low mass (C8H9NO5) drug, which is used to protect penicillins and other β-lactam antibiotics from hydrolysis by serine β-lactamases. As shown by detailed mass spectrometric analyses, clavulanic acid reacts covalently with serine β-lactamase to form an initial acyl-enzyme complex which, like the analogous complex formed with β-lactam antibiotics, can undergo hydrolysis. In the case of clavulanic acid, however, the acyl-enzyme complex also undergoes competing fragmentation to give inhibitory complexes stable to hydrolysis.