Scientists at the Ineos Oxford Institute (IOI) have found a new potential combination therapy to combat antimicrobial resistance (AMR) by targeting two key bacterial enzymes involved in resistance.
Meropenem is a critical antibiotic used to treat serious multidrug-resistant infections like sepsis when other antibiotics such as penicillin have failed. However, this last resort drug is becoming less effective at treating infections due to antimicrobial resistance, or AMR.
One effective strategy to restore the activity of the antibiotic is to use a combination therapy to counter bacterial resistance mechanisms. An antibiotic combination treatment includes an antibiotic and an inhibitor. The inhibitor prevents bacterial enzymes such as metallo- β -lactamases (MBLs) and serine-β-lactamases (SBLs) from breaking down the antibiotic before it has its desired effect to treat the infection.
Research to date has largely focused on developing SBL inhibitors and these are now widely used in clinics and hospitals. Scientists at the IOI are developing new MBL inhibitors to be used in combination therapies.
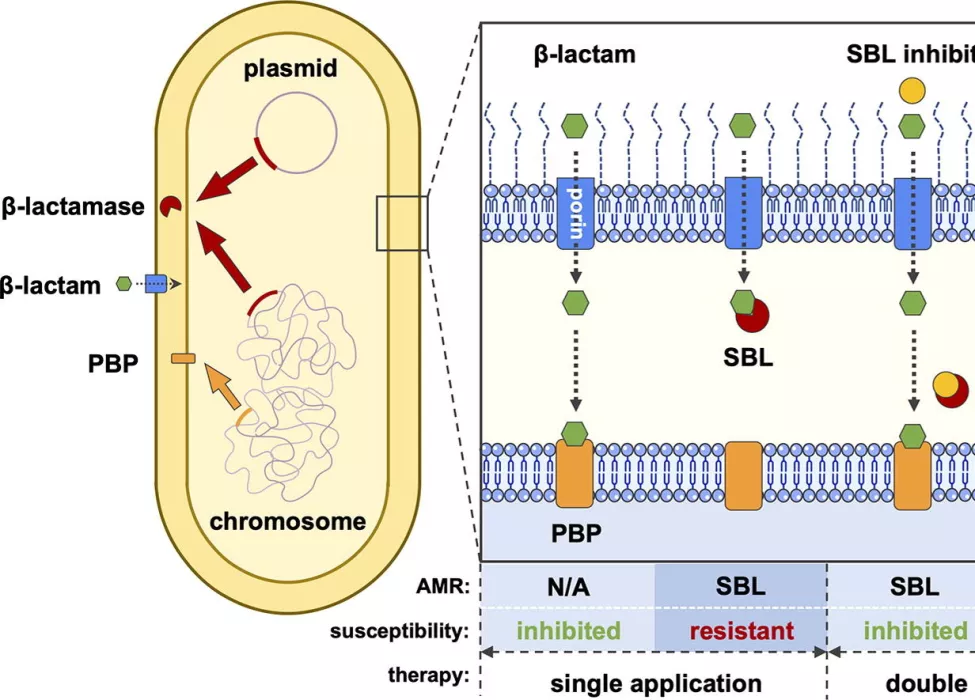
This new study looked at a combination of three drugs: the β-lactam antibiotic meropenem, a newly developed MBL inhibitor called indole-2-carboxylate 58 (InC58), and an SBL inhibitor called avibactam (AVI).In hospital settings, it is difficult to determine whether a strain of bacteria causing an infection is producing SBLs or MBLs or if it is harboring both resistance mechanisms. This is the first study to examine the combination of a carbapenem antibiotic with two inhibitors targeting SBLs and MBLs separately.
The team tested the effectiveness of the combination of all three compounds, compared to a combination of meropenem with either InC58 or AVI alone, on 51 strains of meropenem-resistant bacteria.
Researchers compared minimum inhibitory concentration (MIC) of the different drug combinations. The MIC is the lowest concentration of a drug that is able to prevent the visible growth of a bacterial strain. An antibiotic with a low MIC value is more effective than one with a high MIC.
The study found that the triple-drug combination was more effective at stopping growth of bacteria in the lab than either of the dual-drug combinations. The combination of meropenem with InC58 and AVI at a concentration of 4 mg/L lowered the MIC50 against all the bacterial isolates tested to 0.5 mg/L. This was 64 times lower than the MIC50 of meropenem combined with AVI alone (32 mg/L) and 4 times lower than the MIC50 of meropenem combined with InC58 alone (2 mg/L). This demonstrates a broad spectrum of antibacterial activity against different strains of MBL- and SBL-producing bacteria.
This study builds on our previous work to develop broad spectrum metallo β-lactamase inhibitors. Here we combatted multiple resistance mechanisms simultaneously to great effect, and this is a great example of how chemistry and microbiology teams can collaborate to develop new potential therapies. This combination therapy works very well in the lab and the next challenge will be to show that this works in infection models and ultimately in a hospital setting.

Genetic analysis was done on the bacterial mutants which showed resistance to the effects of the new InC58 and meropenem combination. Resistance was correlated with mutations in two genes associated with changes to porins (channels on bacterial outer membrane) and copper permeability in bacteria. This information helps scientists to understand how resistance to new drug combinations including MBL inhibitors like InC58 could develop in the future.
These findings suggest a potential new combination therapy for meropenem-resistant infections, and whilst this works very well in the lab further development is needed to show that this may also be effective in a hospital setting. The findings provide a benchmark for the activity of a single ideal molecule which can evade the resistance mechanisms. Such novel treatments could significantly extend the antibacterial activity of carbapenems, and possibly other β-lactam antibiotics.
